How To Find Lone Pairs Of An Element

In chemistry, a lonely pair refers to a pair of valence electrons that are not shared with some other atom in a covalent bail[i] and is sometimes called an unshared pair or non-bonding pair. Lonely pairs are found in the outermost electron shell of atoms. They can be identified by using a Lewis construction. Electron pairs are therefore considered lone pairs if ii electrons are paired but are not used in chemical bonding. Thus, the number of lone pair electrons plus the number of bonding electrons equals the total number of valence electrons effectually an cantlet.
Lone pair is a concept used in valence shell electron pair repulsion theory (VSEPR theory) which explains the shapes of molecules. They are also referred to in the chemistry of Lewis acids and bases. However, not all non-bonding pairs of electrons are considered past chemists to exist lonely pairs. Examples are the transition metals where the non-bonding pairs do not influence molecular geometry and are said to be stereochemically inactive. In molecular orbital theory (fully delocalized canonical orbitals or localized in some form), the concept of a lone pair is less singled-out, as the correspondence betwixt an orbital and components of a Lewis construction is ofttimes not straightforward. All the same, occupied not-bonding orbitals (or orbitals of more often than not nonbonding graphic symbol) are ofttimes identified as lone pairs.
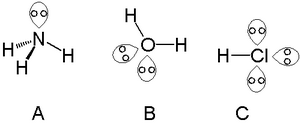
A single lone pair can be found with atoms in the nitrogen group such as nitrogen in ammonia, two lone pairs tin be plant with atoms in the chalcogen group such as oxygen in water and the halogens tin can carry 3 lone pairs such as in hydrogen chloride.
In VSEPR theory the electron pairs on the oxygen cantlet in water course the vertices of a tetrahedron with the alone pairs on two of the four vertices. The H–O–H bond angle is 104.five°, less than the 109° predicted for a tetrahedral bending, and this can be explained past a repulsive interaction betwixt the alone pairs.[ii] [3] [4]
Diverse computational criteria for the presence of lone pairs accept been proposed. While electron density ρ(r) itself generally does not provide useful guidance in this regard, the laplacian of the electron density is revealing, and 1 benchmark for the location of the lone pair is where L(r) = –∇iiρ(r) is a local maximum. The minima of the electrostatic potential V(r) is another proposed criterion. Even so another considers the electron localization function (ELF).[5]
Bending changes [edit]

Tetrahedral Structure of Water
The pairs oft exhibit a negative polar character with their high accuse density and are located closer to the atomic nucleus on boilerplate compared to the bonding pair of electrons. The presence of a solitary pair decreases the bond angle between the bonding pair of electrons, due to their high electric charge which causes great repulsion between the electrons. They are also used in the formation of a dative bond. For case, the creation of the hydronium (H3O+) ion occurs when acids are dissolved in water and is due to the oxygen atom altruistic a lone pair to the hydrogen ion.
This can be seen more clearly when looked at it in two more common molecules. For example, in carbon dioxide (COtwo), the oxygen atoms are on reverse sides of the carbon, whereas in h2o (H2O) there is an angle between the hydrogen atoms of 104.5º. Due to the repulsive force of the oxygen cantlet'south lone pairs, the hydrogens are pushed further away, to a signal where the forces of all electrons on the hydrogen atom are in equilibrium. This is an illustration of the VSEPR theory.
Dipole moments [edit]
Solitary pairs can brand a contribution to a molecule'due south dipole moment. NH3 has a dipole moment of one.47 D. Equally the electronegativity of nitrogen (3.04) is greater than that of hydrogen (2.2) the result is that the Northward-H bonds are polar with a net negative charge on the nitrogen cantlet and a smaller internet positive charge on the hydrogen atoms. In that location is also a dipole associated with the lonely pair and this reinforces the contribution fabricated by the polar covalent Northward-H bonds to ammonia's dipole moment. In dissimilarity to NH3, NFthree has a much lower dipole moment of 0.24 D. Fluorine is more electronegative than nitrogen and the polarity of the N-F bonds is contrary to that of the N-H bonds in ammonia, and so that the dipole due to the lonely pair opposes the N-F bond dipoles, resulting in a low molecular dipole moment.[6]
Stereogenic lone pairs [edit]
A lone pair can contribute to the existence of chirality in a molecule, when three other groups attached to an atom all differ. The effect is seen in certain amines, phosphines,[7] sulfonium and oxonium ions, sulfoxides, and even carbanions.
The resolution of enantiomers where the stereogenic centre is an amine is commonly precluded because the energy bulwark for nitrogen inversion at the stereo middle is depression, which allow the two stereoisomers to rapidly interconvert at room temperature. Equally a result, such chiral amines cannot be resolved, unless the amine's groups are constrained in a cyclic structure (such as in Tröger'due south base).
Unusual solitary pairs [edit]
A stereochemically active lone pair is also expected for divalent lead and tin ions due to their formal electronic configuration of due northsouthward 2. In the solid state this results in the distorted metal coordination observed in the litharge structure adopted by both PbO and SnO. The formation of these heavy metal due norths ii alone pairs which was previously attributed to intra-atomic hybridization of the metallic s and p states[8] has recently been shown to accept a strong anion dependence.[9] This dependence on the electronic states of the anion can explain why some divalent lead and tin materials such as PbS and SnTe evidence no stereochemical bear witness of the lonely pair and adopt the symmetric rocksalt crystal structure.[x] [11]
In molecular systems the lone pair can also result in a distortion in the coordination of ligands around the metal ion. The lead lone pair upshot tin can be observed in supramolecular complexes of lead(2) nitrate, and in 2007 a written report linked the lone pair to lead poisoning.[12] Lead ions can replace the native metal ions in several key enzymes, such as zinc cations in the ALAD enzyme, which is also known every bit porphobilinogen synthase, and is important in the synthesis of heme, a central component of the oxygen-conveying molecule hemoglobin. This inhibition of heme synthesis appears to be the molecular ground of lead poisoning (also called "saturnism" or "plumbism").[thirteen] [14] [15]
Computational experiments reveal that although the coordination number does not change upon commutation in calcium-binding proteins, the introduction of pb distorts the way the ligands organize themselves to suit such an emerging lone pair: consequently, these proteins are perturbed. This lone-pair effect becomes dramatic for zinc-bounden proteins, such every bit the above-mentioned porphobilinogen synthase, as the natural substrate cannot bind anymore - in those cases the poly peptide is inhibited.
In Grouping 14 elements (the carbon group), alone pairs tin can manifest themselves by shortening or lengthening single (bond order 1) bond lengths,[16] as well equally in the effective order of triple bonds besides.[17] [18] The familiar alkynes have a carbon-carbon triple bond (bail order 3) and a linear geometry of 180° bond angles (figure A in reference [19]). However, further down in the group (silicon, germanium, and tin), formal triple bonds have an effective bond order two with one lone pair (figure B [19]) and trans-aptitude geometries. In pb, the effective bail order is reduced even farther to a single bail, with two lone pairs for each atomic number 82 atom (figure C [xix]). In the organogermanium compound (Scheme 1 in the reference), the effective bond club is also 1, with complexation of the acidic isonitrile (or isocyanide) C-North groups, based on interaction with germanium's empty 4p orbital.[xix] [twenty]

Unlike descriptions for multiple lonely pairs [edit]
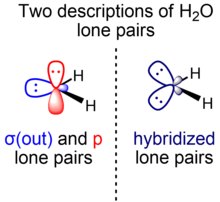
The symmetry-adapted and hybridized alone pairs of HtwoO
In elementary chemistry courses, the lone pairs of h2o are described as "rabbit ears": two equivalent electron pairs of approximately sp3 hybridization, while the HOH bail bending is 104.5°, slightly smaller than the ideal tetrahedral angle of arccos(–1/3) ≈ 109.47°. The smaller bond angle is rationalized past VSEPR theory by ascribing a larger space requirement for the 2 identical lone pairs compared to the two bonding pairs. In more advanced courses, an alternative explanation for this phenomenon considers the greater stability of orbitals with excess s grapheme using the theory of isovalent hybridization, in which bonds and alone pairs tin can be synthetic with sp x hybrids wherein nonintegral values of x are allowed, so long equally the full amount of due south and p character is conserved (ane south and three p orbitals in the case of 2d-row p-cake elements).
To make up one's mind the hybridization of oxygen orbitals used to form the bonding pairs and lone pairs of water in this movie, we use the formula 1 + x cos θ = 0, which relates bond angle θ with the hybridization alphabetize x. According to this formula, the O–H bonds are considered to be constructed from O bonding orbitals of ~sp4.0 hybridization (~80% p character, ~20% s grapheme), which leaves behind O lone pairs orbitals of ~sp2.three hybridization (~lxx% p character, ~30% south character). These deviations from idealized sp3 hybridization for tetrahedral geometry are consistent with Bent's dominion: lone pairs localize more electron density closer to the central atom compared to bonding pairs; hence, the use of orbitals with excess s graphic symbol to form lone pairs (and, consequently, those with excess p graphic symbol to form bonding pairs) is energetically favorable.
However, theoreticians often adopt an culling clarification of h2o that separates the lone pairs of h2o co-ordinate to symmetry with respect to the molecular plane. In this model, there are two energetically and geometrically distinct lone pairs of water possessing dissimilar symmetry: ane (σ) in-plane and symmetric with respect to the molecular airplane and the other (π) perpendicular and anti-symmetric with respect to the molecular plane. The σ-symmetry lone pair (σ(out)) is formed from a hybrid orbital that mixes 2s and 2p character, while the π-symmetry lone pair (p) is of exclusive 2p orbital parentage. The s graphic symbol rich O σ(out) lone pair orbital (also notated northward O (σ)) is an ~sp0.seven hybrid (~xl% p character, 60% s character), while the p lone pair orbital (also notated northward O (π)) consists of 100% p character.
Both models are of value and represent the aforementioned total electron density, with the orbitals related by a unitary transformation. In this case, we can construct the two equivalent solitary pair hybrid orbitals h and h' by taking linear combinations h = c 1σ(out) + c 2p and h' = c 1σ(out) – c 2p for an appropriate choice of coefficients c 1 and c 2. For chemical and physical properties of water that depend on the overall electron distribution of the molecule, the utilise of h and h' is merely as valid as the utilize of σ(out) and p. In some cases, such a view is intuitively useful. For case, the stereoelectronic requirement for the anomeric upshot can exist rationalized using equivalent lone pairs, since it is the overall donation of electron density into the antibonding orbital that matters. An alternative treatment using σ/π separated alone pairs is also valid, only information technology requires striking a balance betwixt maximizing n O (π)-σ* overlap (maximum at xc° dihedral angle) and northward O (σ)-σ* overlap (maximum at 0° dihedral bending), a compromise that leads to the conclusion that a gauche conformation (60° dihedral angle) is most favorable, the same conclusion that the equivalent solitary pairs model rationalizes in a much more straightforward manner.[21] Similarly, the hydrogen bonds of water form forth the directions of the "rabbit ears" alone pairs, as a reflection of the increased availability of electrons in these regions. This view is supported computationally.[5] However, because only the symmetry-adjusted canonical orbitals have physically meaningful energies, phenomena that accept to do with the energies of individual orbitals, such as photochemical reactivity or photoelectron spectroscopy, are about readily explained using σ and π lone pairs that respect the molecular symmetry.[21] [22]
Considering of the popularity of VSEPR theory, the treatment of the water lonely pairs every bit equivalent is prevalent in introductory chemical science courses, and many practicing chemists continue to regard it as a useful model. A like situation arises when describing the two lone pairs on the carbonyl oxygen of a ketone.[23] Nevertheless, the question of whether it is conceptually useful to derive equivalent orbitals from symmetry-adapted ones, from the standpoint of bonding theory and pedagogy, is nonetheless a controversial one, with contempo (2014 and 2015) articles opposing[24] and supporting[25] the exercise.
See likewise [edit]
| | Wikimedia Commons has media related to Solitary pair. |
- Coordination complex
- Highest occupied molecular orbital
- Inert pair consequence
- Ligand
- Shared pair
References [edit]
- ^ IUPAC Gilded Book definition: lonely (electron) pair
- ^ Fox, Yard.A.; Whitesell, J.K. (2004). Organic Chemical science. Jones and Bartlett Publishers. ISBN978-0-7637-2197-viii . Retrieved 5 May 2021.
- ^ McMurry, J. (2000). Organic Chemistry 5th Ed. Ceneage Learning India Pvt Limited. ISBN978-81-315-0039-2 . Retrieved v May 2021.
- ^ Lee, J.D. (1968). Curtailed Inorganic Chemistry. Student'southward paperback edition. Van Nostrand. Retrieved 5 May 2021.
- ^ a b Kumar, Anmol; Gadre, Shridhar R.; Mohan, Neetha; Suresh, Cherumuttathu H. (2014-01-06). "Lone Pairs: An Electrostatic Viewpoint". The Journal of Concrete Chemistry A. 118 (2): 526–532. Bibcode:2014JPCA..118..526K. doi:10.1021/jp4117003. ISSN 1089-5639. PMID 24372481.
- ^ Housecroft, C. E.; Sharpe, A. M. (2004). Inorganic Chemistry (2nd ed.). Prentice Hall. p. xl. ISBN978-0-13-039913-7.
- ^ Quin, 50. D. (2000). A Guide to Organophosphorus Chemistry, LOCATION: John Wiley & Sons. ISBN 0471318248.
- ^ Stereochemistry of Ionic Solids J.D.Dunitz and L.Eastward.Orgel, Advan. Inorg. and Radiochem. 1960, 2, 1–threescore
- ^ Payne, D. J. (2006). "Electronic Origins of Structural Distortions in Post-Transition element Oxides: Experimental and Theoretical Evidence for a Revision of the Lone Pair Model". Physical Review Letters. 96 (15): 157403. doi:10.1103/PhysRevLett.96.157403. PMID 16712195.
- ^ Walsh, Aron (2005). "The origin of the stereochemically active Lead(II) lonely pair: DFT calculations on PbO and PbS". Journal of Solid Country Chemistry. 178 (5): 1422–1428. Bibcode:2005JSSCh.178.1422W. doi:10.1016/j.jssc.2005.01.030.
- ^ Walsh, Aron (2005). "Influence of the Anion on Lone Pair Formation in Sn(2) Monochalcogenides: A DFT Study". The Periodical of Physical Chemical science B. 109 (xl): 18868–18875. doi:10.1021/jp051822r. PMID 16853428.
- ^ Gourlaouen, Christophe; Parisel, Olivier (15 Jan 2007). "Is an Electronic Shield at the Molecular Origin of Lead Poisoning? A Computational Modeling Experiment". Angewandte Chemie International Edition. 46 (4): 553–556. doi:10.1002/anie.200603037. PMID 17152108.
- ^ Jaffe, Due east. G.; Martins, J.; et al. (13 October 2000). "The Molecular Mechanism of Atomic number 82 Inhibition of Homo Porphobilinogen Synthase". Journal of Biological Chemical science. 276 (2): 1531–1537. doi:10.1074/jbc.M007663200. PMID 11032836.
- ^ Scinicariello, Franco; Murray, H. Edward; et al. (fifteen September 2006). "Lead and δ-Aminolevulinic Acrid Dehydratase Polymorphism: Where Does It Lead? A Meta-Analysis". Ecology Wellness Perspectives. 115 (1): 35–41. doi:x.1289/ehp.9448. PMC1797830. PMID 17366816.
- ^ Chhabra, Namrata (Nov 15, 2015). "Issue of Lead poisoning on heme biosynthetic pathway". Clinical Cases: Biochemistry For Medics. Archived from the original on iii April 2016. Retrieved 30 October 2016.
- ^ Richards, Anne F.; Brynda, Marcin; Power, Philip P. (2004). "Furnishings of the alkali metal counter ions on the germanium–germanium double bond length in a heavier group 14 element ethenide table salt". Chem. Commun. (14): 1592–1593. doi:ten.1039/B401507J. PMID 15263933.
- ^ Power, Philip P. (December 1999). "π-Bonding and the Lone Pair Effect in Multiple Bonds between Heavier Chief Group Elements". Chemic Reviews. 99 (12): 3463–3504. doi:ten.1021/cr9408989. PMID 11849028.
- ^ Vladimir Ya. Lee; Akira Sekiguchi (22 July 2011). Organometallic Compounds of Depression-Coordinate Si, Ge, Sn and Pb: From Phantom Species to Stable Compounds. John Wiley & Sons. p. 23. ISBN978-1-119-95626-6.
- ^ a b c d Spikes, Geoffrey H.; Power, Philip P. (2007). "Lewis base induced tuning of the Ge–Ge bond order in a "digermyne"". Chem. Commun. (1): 85–87. doi:x.1039/b612202g. PMID 17279269.
- ^ Power, Philip P. (2003). "Silicon, germanium, tin and lead analogues of acetylenes". Chemic Communications (17): 2091–101. doi:10.1039/B212224C. PMID 13678155.
- ^ a b A., Albright, Thomas (2013-04-08). Orbital interactions in chemistry. Burdett, Jeremy K., 1947-, Whangbo, Myung-Hwan (2nd ed.). Hoboken, New Jersey. ISBN9780471080398. OCLC 823294395.
- ^ While n O(π) solitary pair is equivalent to the canonical MO with Mulliken label aneb ane, the n O(σ) lone pair is non quite equivalent to the canonical MO of Mulliken label iia 1, since the fully delocalized orbital includes mixing with the in-stage symmetry-adapted linear combination of hydrogen 1s orbitals, making it slightly bonding in character, rather than strictly nonbonding.
- ^ Ansyln, Eastward. 5.; Dougherty, D. A. (2006). Modern Physical Organic Chemical science . Sausalito, CA: University Scientific discipline Books. pp. 41. ISBN978-i-891389-31-3.
- ^ Clauss, Allen D.; Nelsen, Stephen F.; Ayoub, Mohamed; Moore, John W.; Landis, Clark R.; Weinhold, Frank (2014-x-08). "Rabbit-ears hybrids, VSEPR sterics, and other orbital anachronisms". Chemistry Education Research and Practice. 15 (4): 417–434. doi:10.1039/C4RP00057A. ISSN 1756-1108.
- ^ Hiberty, Philippe C.; Danovich, David; Shaik, Sason (2015-07-07). "Comment on "Rabbit-ears hybrids, VSEPR sterics, and other orbital anachronisms". A reply to a criticism". Chemical science Education Inquiry and Do. 16 (3): 689–693. doi:10.1039/C4RP00245H. S2CID 143730926.
Source: https://en.wikipedia.org/wiki/Lone_pair
Posted by: coatesperis1986.blogspot.com


0 Response to "How To Find Lone Pairs Of An Element"
Post a Comment